AHA Rapid Response Grant COVID-19 and its Cardiovascular Impact
Background
At the beginning of the pandemic in March 2020, the American Heart Association issued an unprecedented rapid response call for cardiovascular/cerebrovascular research proposals to address the growing crisis of the COVID-19 pandemic. Because of the urgency of this issue, the focus was on innovative, highly impactful short-term proposals (9-12 months) that could show progress within the award period. Approximately 750 proposals were prepared over a two-week period and about 150 volunteers stepped forward to review them. The AHA funded 17 awards, each ranging from $75,000 to $200,000, and a nationwide Coordinating Center.
In addition, the four centers that comprise the AHA Health Technologies & Innovation Strategically Focused Research Network received supplemental funding for short-term special projects to focus on rapid technology solutions to address the COVID-19 pandemic crisis.
AHA Rapid Response Grant COVID-19 and its Cardiovascular Impact RFA
Video Reports from the Awardees
Risk of Severe Morbidity and Mortality of Coronavirus Disease 2019 (COVID-19) Among Patients Taking Antihypertensive Medications
presented by Jaejin An, PhD
Kaiser Permanente Southern California
The Role of the Platelet in Mediating Cardiovascular Disease in SARS-CoV-2 Infection
presented by Milka Koupenova, PhD
on behalf of Jane Freedman
University of Massachusetts Medical School
Echocardiogram to Predict the Prognosis of Patients Admitted with COVID-19
presented by Bruno R. Nascimento, MD, MSc, PhD, FACC, FESC - UFMG School of Medicine
on behalf of Andrea Z. Beaton
Cincinnati Children's Hospital
AHA COVID-19 Rapid Response Grant Research Teams
Open each section for project overview and progress updates
Open each section for project overview and progress updates
Selected to serve as the COVID-19 Coordinating Center, this team collects results from the research projects and coordinates the dissemination of all study findings.
PROGRESS UPDATES:
3/26/22: The awarded researchers met virtually every 4-6 weeks and gathered in Cleveland, Ohio, March 25-26, 2022, in a hybrid meeting to share and discuss research results. The breadth and depth of the research funded by AHA were evident throughout the meeting and included clinical, translational and basic studies yielding insights into SARS-CoV-2 infection, pathophysiology and management. Projects included clinical trials on clinical management of COVID-19, including randomized trials on steroid and anti-thrombotic therapies, novel data collection and analytic methods, and telemedicine/tele-research approaches, to collection of autopsy, heart biopsy, and blood samples, to basic labs pivoting to work on SARS-CoV-2 infection. Throughout all the projects, researchers overcame structural and practical barriers, demonstrating the hard work and dedication of researchers operating in the throes of the pandemic. Most rapid-response grants continued to be active through a no cost extension, and the SFRN supplements remain active.
“Beyond the scientific yield . . . the program provided a conduit for cardiovascular investigators to immediately engage in the battle against this serious and unprecedente health threat. On behalf of my research team . . . thank you AHA for what you've done.” —Michael Bristow, MD, PhD. Professor, Medicine-Cardiology, University of Colorado School of Medicine
Scientific Sessions 2021: Rapid Fire Results From AHA Research on COVID-19
This video recording highlights results from the COVID-19 research awardees in a rapid-fire format.
Presentation date: November 14, 2021
AHA Rapid Response COVID-19 Research – What's Hot - Nov. 11, 2020 (PPT)
Columbia University Irving Medical Center, led by Sanjum S. Sethi, M.D., M.P.H.
Working with New York-Presbyterian and the Cardiovascular Research Foundation, this team will evaluate the clotting complications of COVID-19 in hospitalized patients, develop a risk score to aid in bedside decision-making and conduct a clinical trial to determine optimal medical treatment to prevent clotting complications in future COVID-19 patients.
Overall Project Aims:
Given the association between COVID-19 critical illness and thrombotic events the aim of this study is to provide an overview of the natural history of both the arterial and venous thrombotic complications in this disease process stratified by ICU and non-ICU patients, to develop a clinical risk score to aid with clinical decision making and risk stratification, and to conduct a randomized controlled trial comparing low dose versus intermediate dose anticoagulation in COVID -19 patients.
Findings:
Sethi Thrombotic Complications in COVID 19 Slides 10 16 20 (PDF)
Publication:
Discordance in activated partial thromboplastin time and anti-factor Xa levels in COVID-19 patients on heparin therapy
Abnormal coagulation parameters and thrombotic complications are common in coronavirus disease 2019 (COVID-19) patients and are associated with poor outcomes. Disruption of the activated partial thromboplastin time (aPPT), also shown to be common in this population, making aPTT-guided intravenous unfractionated heparin dose adjustment particularly challenging in patients with COVID-19.
Harvard Medical School, led by Joseph Loscalzo, M.D., Ph.D.
Working with the Center for Complex Network Research of Northeastern University, Lawrence Livermore National Laboratory and the National Emerging Infectious Diseases Laboratory at Boston University, this team will look at repurposing already approved drugs for faster applications in treating COVID-19 patients.
Overview:
This study looked at whether or not we can identify current drugs that could also help in treating Covid-19. Using network medicine approaches, investigators identified pathways that govern the effect of SARS-CoV-2 on the heart and human heart cells, identified potential drug targets that disrupt the interaction of the virus with the heart, and identified repurposable approved drugs that could interact with these targets for use in Covid-19. Investigators identified a subnetwork of human proteins that interact with SARS-CoV-2 proteins (the “covidome”) and showed that the covidome overlapped with other disease modules related to conditions associated with worse SARS-CoV-2 infections (i.e. the obesity and the cerebrovascular disease modules). Investigators did find drugs and confirmed many of these in cell screening tests and animal models (mice that express human ACE2, the host cell receptor for SARS-CoV-2 virus). They showed drugs that helped stop the virus and Covid-19 from growing and infecting cells, suggesting these drugs may benefit from more testing against Covid-19.
Publications:
Patten, J.J., Keiser, P.T., Gysi, D., Menichetti, G., Mori, H., Donahue, C.J., Gan, X., Do Valle, I., Geoghegan-Barek, K., Anantpadma, M., Berrigan, J.L., Jalloh, S., Ayazika, T., Wagner, F., Zitnik, M., Ayehunie, S., Anderson, D., Loscalzo, J., Gummuluru, S., Manchuk, M.N., Barabasi, A.L., and Davey, R.A. (2021): Multidose evaluation of 6,710 drug repurposing library identifies potent SARS-CoV-2 infection inhibitors In Vitro and In Vivo. bioRxiv 2021.04.20.440626. PMID 33907750
Gysi, D.M., Do Valle, I., Zitnik, M., Ameli, A., Gan, X., Varol, O., Ghiassian S.D., Patten, J.J., Davey, R., Loscalzo, J., and Barabasi, A.-L. (2021): Network medicine framework for identifying drug repurposing opportunities for COVID-19. Proc. Nat’l. Acad. Sci. (USA) 2021;118:e202581118.
Covidome_in_different_tissues_Loscalzo.xlsx



Kaiser Permanente Southern California, led by Jaejin An, Ph.D.
This team will evaluate antihypertensive medication treatment in patients with confirmed COVID-19 infection and high blood pressure and their results could have an immediate impact on clinical guidance for patient care.
Overview:
It was unclear whether the medications for high blood pressure (BP) increased the risk of COVID-19. To properly care people with high BP during the COVID-19 pandemic, this study assessed whether people who take ACE inhibitor (ACEI) or angiotensin receptor blocker (ARB) medications for high BP are at increased risk for COVID-19 infection, hospitalization, and death compared with people who take other medications. In addition, the study looked at the role of BP control in COVID-19 severity.
Findings to date: Overall, the study found that neither ACEI nor ARB use was associated with increased risk of COVID-19 infection. Also, use of ACEIs or ARBs was not associated with an increased risk of hospitalization or death. BP above goal was not associated with hospitalization or death. Instead, people with cardiovascular disease had poor COVID-19 outcomes.
These findings are important: People with high BP should take their medications during the COVID-19 pandemic. This will help minimize the impact of COVID-19 for patients with high BP. While BP control is an important chronic treatment goal, its role in an acute viral illness such as COVID-19 is yet to be determined.
Publications:
An J, Zhou H, Wei R, Luong TQ, Gould MK, Mefford MT, Harrison TN, Creekmur B, Lee MS, Sim JJ, Brettler JW, Martin JP, Ong-Su AL, Reynolds K. COVID-19 morbidity and mortality associated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers use among 14,129 patients with hypertension from a US integrated healthcare system -.Int J Cardiol Hypertens. 2021 Jun;9:100088. doi: 10.1016/j.ijchy.2021.100088. Epub 2021 Jun 15.PMID: 34155486 PMCID: PMC8204813
Angiotensin-Converting Enzyme Inhibitors or Angiotensin Receptor Blockers Use and COVID-19 Infection Among 824 650 Patients With Hypertension From a US Integrated Healthcare System - J Am Heart Assoc. 2021 Feb 2;10(3):e019669. doi: 10.1161/JAHA.120.019669. Epub 2020 Dec 14.
Poster, AHA Scientific Sessions 2020 (PDF)
Disease 2019 (COVID-19) Among Patients Taking Antihypertensive Medications
presented by Jaejin An, PhD
Kaiser Permanente Southern California
March 2022
Massachusetts General Hospital, led by Michael T. Lu, M.D., M.P.H.
Working with the Mass General Brigham hospitals, this team will use deep learning techniques and the initial chest x-ray of patients admitted for treatment to develop a new way to predict COVID-19 cardiopulmonary collapse and death.
Overview:
The study team developed a model using artificial intelligence methods to predict 30-day death risk from a chest X-ray in patients with COVID-19. They have collected the data, trained the CXR-CovRisk model, and conducted much of the analysis. The model appears to perform better than other deep learning models and predicts 30-day mortality and subsequent hospital admissions.
Publications: Manuscript being prepared.
Mayo Clinic, led by Ognjen Gajic, M.D.
This team will expand its current SMART randomized clinical trial to add the evaluation of biomarker-titrated corticosteroids dosing compared to usual care for treating COVID-19 patients.
Overview:
This study addressed the question of whether we can give steroids in a better way to control inflammation in people with COVID-19 disease. The study was a pilot randomized clinical trial to compare usual care against use of a biomarker (C-reactive protein) to adjust steroid.
Progress to Date:
The study showed we can feasibly and safely give steroids based on a blood test that measures the body’s reaction to infection. The study showed a different way of giving steroids to people with COVID-19 disease. A larger multicenter clinical trial is needed to confirm these findings.
Publication: Early, biomarker-guided steroid dosing in COVID-19 Pneumonia: a pilot randomized controlled trial | Critical Care | Full Text (biomedcentral.com)
Stanford University, led by Paul Heidenreich, M.D.
Working with the VA Palo Alto Health Care System, Stanford Health Care and Northern California Kaiser, this team will study the use of ACE and ARBs on patients with high blood pressure or diabetes during the COVID-19 pandemic to determine trends in the rates of COVID-19 infection, influenza, medication adherence, hospitalizations and deaths to improve patient management practices.
Overview:
Working with the VA Palo Alto Health Care System, Stanford Health Care and Northern California Kaiser, this team studied the use of angiotensin converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) use on patients with high blood pressure or diabetes during the COVID-19 pandemic to determine trends in the rates of COVID-19 infection, influenza, medication adherence, hospitalizations and deaths to improve patient management practices.
The study examined how care and outcome for heart disease changed last year during the COVID-19 pandemic. We also examined if certain treatments for high blood pressure protect you from COVID-19. We feel certain blood pressure medications are safe.
Publications:
Sandhu AT, Kohsaka S, Lin S, Woo CY, Goldstein MK, Heidenreich PA. Renin-angiotensin-aldosterone system inhibitors and SARS-CoV-2 infection: an analysis from the veteran's affairs healthcare system. Am Heart J 2021 Jun 12;240:46-57. doi: 10.1016/j.ahj.2021.06.004. Online ahead of print. PMID: 34126079 PMCID: PMC8196226
2 under preparation
University of California, Los Angeles, led by Tzung K. Hsiai, M.D., Ph.D.
Working with the UCLA Cancer Virology Program, California Nanosystems Institute, Statistics and Epidemiology, Data Science, Behavioral Sciences, UCLA Medical Center and West Los Angeles VA Healthcare System, this team will set out to develop the first-of-its-kind COVID-19-on-a-chip, targeting the heart for now, but adaptable to other organ systems impacted by the infection, including the lung, gut, kidney and brain.
Overview:
This project aimed to use a 3-D platform consisting of blood vessel cells exposed to human blood flow to simulate a micro-circulation test system that can be used to find target countermeasures against SARS-CoV-2. The SARS-CoV-2 virus binds to ACE2 and promotes cytokine storm and small vessel clotting (thrombosis). This team made nano-liposomes (very small lipid particles) linked to the Spike protein from SARS-CoV-2 that binds to ACE2. They showed that Spike-protein linked liposomes (“lipo-S”) effectively stimulated mice to make anti-Spike antibodies, affected cardiomyocytes (heart cells), disrupted alveoli (lung tissue) and infiltrated the micro-vasculature (small blood vessels). They found that that lipo-S and other spike protein mutations and accessory proteins increase clotting/thrombosis and showed that anti-IL-6 and decoy Lipo-human ACE2 are target countermeasures that may work in the face of rising spike protein mutations and viral variants.
This work may help to identify new spike protein or new SARS-CoV-2 variants that could cause clotting risk in blood and may help with rapid diagnosis and identification of new therapies for COVID-19.
Publications: in preparation
University of California, San Francisco, led by Michelle A. Albert, M.D., M.P.H.
Working with the UCSF NURTURE Center and the Slone Epidemiology Center at Boston University, this team will use the Black Women’s Health Study cohort to understand the experiences and cardiovascular effects of COVID-19 on African-American women, a population historically at the intersection of the worst health and economic disparities in the United States.
Overview:
This research is seeking to address the effects of the global COVID-19 pandemic on the cardiovascular health and outcomes in African-American women, a demographic at the intersection of the worst health and economic disparities in the United States. In a collaborative effort between investigators at UCSF CeNter for the StUdy of AdveRsiTy and CardiovascUlaR DiseasE (NURTURE Center) and the Slone Epidemiology Center at Boston University, investigators are studying COVID-19 experiences of geographically diverse black women living in the United States and their relationship with cardiovascular and other health outcomes. The study also includes assessment of the role of blood pressure and other medications such as immunosuppressive drugs on disease complications.
Progress thus far includes design and distribution of the BWHS COVID-19 Questionnaire (www.bu.edu/bwhs). By September 2020, 14,000 participants were contacted, and 11,000 women consented to participate. Over 10,000 completed questionnaires were received. In February 2021, COVID-19 vaccination questions were added to the COVID-19 survey and questionnaires were send for a second data collection point; as of June 2021, 9000 completed questionnaires have been received. Data collection, including for longer term information, is ongoing.
The work is extremely significant as it addresses priorities for our nation’s health pertaining to the health of black communities because black women tend to serve as the breadwinners of their communities at physical and psychosocial costs to their well-being. Understanding the impact of COVID-19 on this vulnerable population is critical to 1) understanding the syndemic template related to their experiences and associated health outcomes, including cardiovascular, psychosocial and disability outcomes; 2) helping define where additional resources are needed during the syndemic and beyond; 3) identification of un/under-described experiences and health outcomes unique to or disproportionately affecting black women.
Resulting Publication:
Cozier Y, Castro-Webb N, Hochberg NS, Rosenberg L, Albert MA*, Palmer JR* (* Co-senior authors) Lower serum 25(OH)D levels associated with higher risk of COVID-19 infection in U.S Black women. PLOS ONE 16(7): e0255132. Published July 27, 2021
University of Colorado, led by Michael R. Bristow, M.D., Ph.D.
This team will study the specific mechanisms for how COVID-19 impacts the cardiovascular system either due to a robust inflammatory response and direct myocardial injury because that distinction can define therapeutic treatment.
Overview:
Effects on the heart have emerged as an important component of some critically ill patients with COVID-19 infection, either from myocarditis (inflammation of the heart) or abnormal heart function without obvious inflammatory disease. There were multiple unanswered questions regarding cardiac involvement in SARS CoV-2 disease including: does the virus invade heart cells; if so, does it utilize the same host cellular machinery as in other cells; what are the relative roles of direct tissue injury from the virus vs. from inflammation effects on the heart muscle; what cytokines are active in the heart; and what are the effects on functionally important heart cell genes and gene networks. In order to address these questions, heart muscle biopsy material was obtained with the aim to detect SARS-CoV-2 in cardiac myocytes, determine the degree of inflammatory reaction vs. direct myocardial injury, determine the level of gene expression of the ACE2 binding target and key cellular entry proteases, as well as expression of candidate and global genes compared to non-COVID-19 infected hearts
Progress to date:
In our investigations thus far of COVID-19 and post-vaccine "myocarditis" we have found very little or no evidence of an inflammatory infiltrate or systemic inflammation in the heart, but we have found evidence of blood clotting in small blood vessel nonspecific heart cell changes, as well as gene function abnormalities that could explain both small vessel clotting and heart function abnormalities. However, we need to enroll another 3-4 patients to be certain of these findings.
Publications:
Bristow MR, Zisman LS, Altman NL, Gilbert EM, Lowes BD, Minobe WA, Slavov D, Schwisow JA, Rodriguez EM, Carroll IA, Keuer TA, Buttrick PM, Kao DP. Dynamic Regulation of SARS-Cov-2 Binding and Cell Entry Mechanisms in Remodeled Human Ventricular Myocardium. JACC Basic Transl Sci 2020 Sep;5(9):871-883. doi: 10.1016/j.jacbts.2020.06.007. Epub 2020 Jun 24. PMID: 32838074. PMCID: PMC7314447
University of Massachusetts, led by Jane E. Freedman, M.D.
This team will study the mechanisms of the platelet-mediated immune response of the COVID-19 virus on the cardiovascular system collecting fundamental molecular knowledge about the disease course of infection that will enable development of novel tools and strategies for clinical management that will lead to improved outcomes.
Overview and Findings to Date:
This study addresses why patients who have the infection COVID-19 develop clots in their blood vessels potentially causing strokes and heart attacks. In this project, we studied this question in two ways. We examined patients presenting at the hospital with or without this infection. Next, we studied blood from normal people that we mixed with virus. In some people, the platelets become “sticky” and form clots potentially harming the patients with an occluded blood vessel. Thus, these cells seem to perform two functions and the balance determines whether they are helpful or harmful to an infected patient.
Publications:
Koupenova M, Corkrey HA, Vitseva O, Tanriverdi K, Somasundaran M, Liu P, Soofi S, Bhandari R, Godwin M, Parsi KM, Cousineau A, Maehr R, Wang J, Cameron SJ, Rade JJ, Finberg RW, Freedman JE. SARS-CoV-2 Initiates Programmed Cell Death in Platelets. Circ Res 2021 Jul 23. doi: 10.1161/CIRCRESAHA.121.319117. Online ahead of print. PMID: 34293929
SARS-CoV-2 Initiates Programmed Cell Death in Platelets - Platelets internalize SARS-CoV-2 virions, directly or attached to microparticles, and viral internalization leads to rapid digestion, programmed cell death, and extracellular vesicle release. During COVID-19, platelets mediate a rapid response to SARS-CoV-2 and this response can contribute to dysregulated immunity and thrombosis. (The direct effect of SARS-CoV-2 on platelets has yet to be studied.)
Platelets and Immunity: Going Viral - Activation of certain receptors that mediate a response to degraded viral ssRNA can lead to eventual thrombocytopenia and other symptoms. Removing these viral particles from circulation is a vital part of the immune process, but when dysregulated, it can lead to pathological thrombosis and vessel occlusion.
The Role of the Platelet in Mediating Cardiovascular Disease in SARS-CoV-2 Infection
presented by Milka Koupenova, PhD
on behalf of Jane Freedman
University of Massachusetts Medical School
March 2022
University of Nebraska Medical Center, led by Rebekah L. Gundry, Ph.D.
This group will be exploring specific pathways and biomarkers to identify those most at risk for COVID-19 infection and cardiovascular complications from the virus, and ultimately will look to develop the means for personalized medicine and future genomic testing and treatment.
Overview:
We do not know why some patients have heart problems after COVID-19 infection. Sugars attached to proteins play a role in how we respond to virus infection. Our research hopes to understand how the sugars that are attached to proteins are involved in this process.
Findings to Date:
We have begun to identify sugars that are different in COVID-19 patients. We find changes in sugars in the heart tissue and the blood. The results of our study will provide targets for new therapies to treat patients with COVID-19. The results will also tell us about how the body responds to the virus and how that can lead to heart damage. Analyses are ongoing.
Publication:
Dhawan, Gundry, Brett-Major, Mahr, Thiele, Lindsey, Anderson, COVID-19 and cardiovascular disease: What we know, what we think we know, and what we need to know, Journal of Molecular and Cellular Cardiology, 144:12-14, 2020. PMID: 32339565
Cleveland Clinic, led by Mina Chung, M.D.
This team will use a multidisciplinary approach to 1) study the interaction of SARS-CoV-2 spike protein in cardiac and brain cells, 2) screen possible candidate drugs targeting mechanisms of viral infection in these cells, and 3) assess these drugs using AHA’s COVID-19 registry. Expected outcomes include identification of drugs that can be repurposed and advanced toward clinical trials for treatment of COVID-19
Overview:
We hope to study how the virus for COVID-19 acts on heart and brain cells. This may allow us to test drugs. We will also test drugs in registries of patients with COVID-19. The virus gets into the cells by a receptor molecule called ACE2, helped by other proteins. The heart has high levels of ACE2, and recent reports show that COVID-19 can injure the heart leading to death. Patients with high blood pressure, diabetes, and heart disease are often treated with common drugs like "ACE inhibitors" (name of the drug often ends in "-pril" like captopril, lisinopril, ramipril) and "angiotensin receptor blockers" (name of the drug often ends in "-sartan" like losartan). These drugs may affect ACE2 levels and the concern is that people taking these drugs will have severe disease and even death due to COVID-19, if the drugs increase the levels of ACE2. We are using engineered heart cells that were made from human blood cells that were reprogrammed to stem cells and then made into heart-like cells that beat like the heart. We are also using brain cells to test how virus enters the cells and infects them. By comparing these results to uninfected cells, we will get to know why and how the virus hurts the heart and the brain.
Findings to Date:
In this study we have found that ACE2 is expressed in our heart cell models (cardiomyocytes and engineered heart tissues), but not in the brain cells we tested. A drug that we thought would fool the SARS-Cov2 virus into attaching to ACE2 in the environment and not enter the cell, did not work out. As the heart cells get targeted by more virus, the ACE2 levels change. Results are reassuring so far. Losartan (an ARB) does not increase the ACE2 levels in engineered heart tissues exposed to a form of the virus. Testing these drugs in heart cells helps us understand the safety and even helpfulness of these drugs. So far results show these drugs should be continued even during COVID-19 infection.
This project will give us more information on the safety of drugs, many of which are important drugs for many people. In addition, it may reveal new drugs that can be repurposed for better treatment of COVID-19.
Publications: 1 being prepared
Johns Hopkins University, led by Daniela Cihakova M.D., Ph.D.
This team will seek to identify a potential peripheral biomarker of cardiac inflammation in COVID-19 and address possible mechanisms that lead to cardiac infection and subsequent injury. The results of these studies will also significantly contribute to the knowledge of the immune response in COVID-19 patients.
Overview and Findings to Date:
In COVID-19, blood clotting in small and large vessels of the lungs is one of the most important causes of death. We found mild blood clotting occurs in the hearts of COVID-19 patients, as well as in the lungs. In addition, we studied how COVID-19 causes blood clotting in the hearts. We collected heart samples from the patients who died from COVID-19 and found mild blood clotting in the heart samples. The blood vessels are covered by endothelial cells that inhibit blood clotting in healthy people. Many scientists expected a problem in function of the blood vessels to be the cause the blood clotting in COVID-19. However, no evidence of poor function of blood vessels in COVID-19 has been reported so far. We found endothelial cell changes associated with the blood clotting in the heart samples from COVID-19 patients. Next, we tested if SARS-CoV-2 infects endothelial cells to induce the dysfunction in the hearts. In our study, virus was located outside of blood vessels in the hearts of COVID-19 patients. Therefore, we found no evidence of direct infection of blood vessels by the virus. We confirmed no blood vessels infection by SARS-CoV-2 in in vitro cell culture and infection. To understand how SARS-CoV-2 infects the hearts and causes the poor blood vessels function, we tested which cells contain the entry receptor (ACE2) for the virus. We found a high expression of ACE2 in stroma cells in the heart and confirmed the direct viral infection in vitro.
Thrombomodulin is one of the blood clotting inhibitors expressed in the blood vessels. In COVID-19 patient's hearts and lungs, the poor functioning blood vessels lost this molecule. Thus, a recombinant thrombomodulin treatment could be considered as a potential therapy to prevent dangerous thrombosis during COVID-19.
Publications: In preparation
Cedars-Sinai Medical Center, co-led by Clive Svendsen, Ph.D. and by Arun Sharma, Ph.D.
This team will investigate whether SARS-CoV-2 can directly infect human cardiomyocytes, assess resulting effects and seek to establish a cardiomyocyte-specific antiviral drug screening platform against SARS-CoV-2. The findings potentially may lead to development of novel therapeutics for the cardiac-specific effects of COVID-19.
Overview:
COVID-19 has caused the hospitalization of hundreds of thousands of people worldwide. Patients show lung symptoms, including cough, and shortness of breath. However, COVID-19 could also cause heart problems such as rhythm problems and heart failure. Heart injury is associated with increased risk of death. It is worth studying whether the virus can directly infect the heart. This will help develop new therapies against COVID-19. Investigators made human heart cells in a dish from stem cells and used these cells to show that human heart cells can be directly infected by the coronavirus. Investigators also developed a drug screening system that can identify new drugs against COVID-19. They detected key proteins involved in pathways critical to SARS-CoV-2 infection and demonstrated potent antiviral activity using berzosertib. In summary, this study helped to understand the mechanisms of SARS-CoV-2 infection, and to develop novel antiviral approaches for alleviating COVID-19.
Publications:
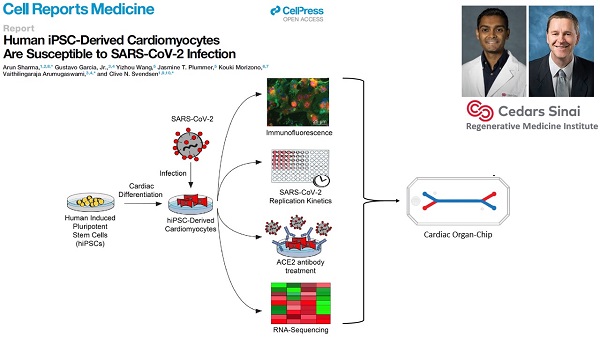
Garcia G Jr, Sharma A, Ramaiah A, Sen C, Purkayastha A, Kohn DB, Parcells MS, Beck S, Kim H, Bakowski MA, Kirkpatrick MG, Riva L, Wolff KC, Han B, Yuen C, Ulmert D, Purbey PK, Scumpia P, Beutler N, Rogers TF, Chatterjee AK, Gabriel G, Bartenschlager R, Gomperts B, Svendsen CN, Betz UAK, Damoiseaux RD, Arumugaswami V. Antiviral drug screen identifies DNA-damage response inhibitor as potent blocker of SARS-CoV-2 replication. Cell Rep. 2021 Apr 6;35(1):108940. doi: 10.1016/j.celrep.2021.108940. Epub 2021 Mar 18. PMID: 33784499. (PDF)
A follow-up to the 2020 Cell Reports Medicine hiPSC-cardiomyocyte study. The group has identified, via a high-throughput screen, some kinase inhibitors that can reduce SARS-CoV-2 proliferation in hiPSC-cardiomyocytes and other cell types.
Columbia University Vagelos College of Physicians & Surgeons, led by Emily J. Tsai
This team will compare single nuclei RNA sequences and histopathological findings between the hearts of COVID-19 non-survivors with and without suspected COVID-19 cardiac disease. Understanding the viral and host factors that drive cardiac injury in COVID-19 will help determine appropriate therapeutic strategies.
Overview:
Although Covid-19 is mostly thought of as a viral pneumonia, it can also effect the heart. How the Covid-19 virus (SARS-CoV-2) injures the heart is not well understood. One popular theory is that the virus infects the heart and the immune response then causes damage to the heart. This project aimed to determine how SARS-CoV-2 injures the heart. We examined hearts of patients who died from Covid-19. We tested whether certain abnormal findings in the heart tissue could be associated with the virus.
We did studies to determine whether the virus was present in the heart tissue samples. We also examined the relationships between abnormal tissue findings and patient clinical data. And lastly we determined the RNA sequence of each and every cell in a subgroup of the Covid-19 hearts. We compared the cell type specific RNA sequences of the Covid-19 hearts with that of hearts from organ donors who did not have Covid-19. Our findings of this analysis help us define Covid-19 heart injury. It also shows how different cardiac cells communicate with each other and damage the heart. The details of our findings can help identify ways to treat or prevent damage to the heart in Covid-19 patients.
Publication: 1 submitted
Pre-print:
Fukuma N, Hulke ML, Brener MI, Golob S, Zilinyi R, Zhou Z, Tzimas C, Russo I, McGroder C, Pfeiffer R, Chong A, Zhang G, Burkhoff D, Leon MB, Maurer M, Moses JW, Uhlemann AC, Hibshoosh H, Uriel N, Szabolcs MJ, Redfors B, Marboe CC, Baldwin MR, Tucker NR, Tsai EJ. Molecular Pathophysiology of Cardiac Injury and Cardiac Microthrombi in Fatal COVID-19: Insights from Clinico-histopathologic and Single Nuclei RNA Sequencing Analyses. bioRxiv 2021 Jul 27;2021.07.27.453843. doi: 10.1101/2021.07.27.453843. Preprint. PMID: 34341789 PMCID: PMC8328056
University of Texas Health Science Center at San Antonio, led by Anand Prasad, M.D.
Overview:
The goal of this study is to determine the incidence, degree, risk factors and clinical outcomes of new onset cardiac dysfunction in patients infected with the SARS-CoV-2 Virus defined by elevation in cardiac biomarkers and ventricular systolic and diastolic dysfunction on echocardiography. The study is in progress.
AHA Rapid Response Supplemental Grants
Along with the new rapid response research grants above, the American Heart Association is also investing $800,000 in supplemental, short-term special projects at the four centers in its Health Technologies & Innovation Strategically Focused Research Network. This funding is in addition to their original grants and will focus on rapid technology solutions to address the COVID-19 pandemic crisis.
Cincinnati Children's Hospital, led by Andrea Z. Beaton, M.D.
Recently, automated intelligence, or computer learning, has become available to help non-expert users take better ultrasound pictures of the heart (echocardiogram) and even provide them with an automatic interpretation. One such software, Navigational Guidance, directs users to the correct position, automatically captures the best images, and provides an automatic value for the squeeze of the heart, known as the ejection fraction. Tools such as these, create the potential for more routine integration of heart imaging into clinical care.
This study takes a broad look at the value of integrating routine measurement of ejection fraction in the setting of COVID-19 from both the patient and provider perspective. Navigational guidance will be tested in the Emergency Department and Intensive Care Unit facing COVID-19. The experience and performance of frontline providers will be measured. Detailed clinical data, including ejection fraction, will be collected on a large group of patients with COVID-19. This data will be used to develop a risk prediction score, to improve the early identification of patients most likely to become critically ill.
Overview:
Coronavirus infections can be mild or severe. It can be hard for doctors and nurses to know which patients will have severe illness when they first come to a hospital. This challenge is even greater in areas of the country and world that do not have access to advanced testing. Echocardiography is a bedside test that can see how the heart is squeezing. This project is looking at the role of echocardiography to predict risk of severe illness in patients who might have coronavirus. Five hospitals in the United States and one hospital in Brazil have contributed over 600 cases to our study. Our team will using these cases to develop a risk score to help improve care for patients who might have coronavirus around the world.
Ejection Fraction as the Sixth Vital Sign for Patients with COVID (PPT)
Echocardiogram to Predict the Prognosis of Patients Admitted with COVID-19
presented by Bruno R. Nascimento, MD, MSc, PhD, FACC, FESC
UFMG School of Medicine
on behalf of Andrea Z. Beaton
Cincinnati Children's Hospital
March 2022
Johns Hopkins University, led by David Newman-Toker, M.D.
Background:
Strokes can have obvious and crippling symptoms, such as paralysis on one side of the body or trouble speaking. Strokes can also have milder symptoms like dizziness or vertigo, which can be mistaken for common inner ear disorders. Prompt stroke diagnosis can lead to treatments that restore brain blood flow, prevent stroke worsening, and limit or reverse disability. It can also decrease the risk of future stroke and heart attack. However, fear of catching COVID-19 stopped people with early stroke symptoms like dizziness from going to the hospital.
Specialist doctors can tell the difference between brain and ear problems by looking at a patient’s eye movements with special goggles that record and measure eye movements of dizzy patients. This is more accurate than brain imaging such as CT or MRI. Looking at eye movements remotely requires high-quality video. This cannot be done with routine streaming video used in telemedicine services. This American Heart Association-funded project will create equitable access to specialists through mobile tele-diagnosis. Our solution is to create a smartphone app that will record patient eye movements while patients are at home. The videos will be sent to our patient database where specially trained doctors will review the videos. Urgent patients will be directed to the nearest hospitals because of concern about stroke. Not urgent patients will be scheduled for a virtual appointment.
Progress to Date:
In this reporting period, we have created a smartphone app from scratch. The app works with android and IOS phones. The next step is to place the app in the hands of patients to review and comment on the design. Our team worked closely with the patient call center. The call center staff will provide patients access to the smartphone app using a secure desktop software tool. Both software tools were created from scratch.
Smartphones are common and can reduce disparity in health care. While at home, patients can use smartphones to connect with specially trained doctors without going to the hospital. Future smartphone apps will replace the need for a specially trained doctor. The app will provide diagnosis and treatment.
University of Michigan, led by Brahmajee Nallamothu
Background:
Our study group was interested in determining how the pandemic (and COVID 19 infection) has affected important feelings of mood and stress, as well as measurable activity levels like step count. We approached studying these important topics in two ways:
We looked at an existing study, called MIPACT, where participants wore an Apple Watch, answered weekly survey questions about their health weekly, took their blood pressure at home, and agreed for the study team to review their health records. We were able to follow these patients through the pandemic, from December 2019 to September 2020. We showed mood and stress scores changed a lot when the pandemic started, and those changes continued over time. We saw this across different races and age groups when we looked at mood and stress over time for these groups. We saw that mood scores in the African American group appeared to be higher compared to other races. Step counts dipped when the first COVID cases were reported, then appeared to return to normal. We still have to look this data more closely before we make any conclusions. Our hope is that understanding the impact of the pandemic on a person’s activity levels, mood, and stress, and especially if they were infected with COVID, will enable us to be better prepared for the next pandemic.
We asked student athletes from the University of Michigan who recently had COVID 19 to join a study that tracked activity, heart rate, clinical symptoms, and mood and stress during both the early and later phases of the infection. We asked each of these student athletes to wear an Apple Watch that we gave them, as well as answer survey questions that we sent to them on their phones. For the first two weeks of the study, these questions were sent daily, then weekly for an additional 10 weeks. The total duration of the study was 90 days. We started enrolling patients in February 2021, and we will finish at the end of May 2021. Our very early review showed differences in heart rate and step count by sex and sport, but more detailed review is necessary to draw any conclusions. We hope to better understand the health of these highly fit student athletes who suffered from COVID 19 infections so that safe recovery protocols can be implemented for this young group.
Stanford University, led by Paul Wang, M.D.
The novel coronavirus (SARS-CoV-2) rapidly became a global pandemic. To capture scientific information about the novel coronavirus, the American Heart Association has rapidly created the COVID-19 Get With The Guidelines® (GWTG) registry. Obtaining such data will be critical in advancing our knowledge about the novel coronavirus and developing effective strategies to combat it. Entering the data can be resource intensive and be a barrier for inclusion of centers that have particularly diverse and underserved populations. We, therefore, propose to develop a set of tools that will simplify data entry and have several benefits: 1) decrease burden of data capture, particularly during surges in hospital resource use; 2) permit analysis of data during the pandemic; 3) avoid bias of omitting entry of complicated long length of stay admissions; 4) encouraging low resource institutions to participate, reducing disparities in populations studied.
Overview:
The study wants to find a better way to get hospitals to enter data when they do not have enough people to do so. We have been able to create a study that will show this can be done. This will help AHA in getting more hospitals to enter data. Hospitals with fewer people for data entry may be helped the most.
Major Findings to Date
- Blood pressure medication is safe to take and does not increase risk of COVID infection, hospitalization or death. This includes the use of ACEIs and ARBs and was confirmed in two large studies (see studies by Jaejin An and Paul Heidenreich).
- Cardiac tissue studies show little to no evidence of inflammation in the hearts biopsied or studied, but there was evidence of more frequent microvascular thrombosis and evidence of injury (see studies by Michael Bristow, Emily Tsai). Platelet and endothelial dysfunction have been implicated in these AHA-funded studies (see Jane Freedman and Daniela Cihakova).
- New human stem-cell-derived heart cell models have been used to study ACE2 and SARS-CoV-2 infection. These have been used to study virus effects and test drugs (see Tzung Hsiai, Clive Svendsen, Mina Chung). Also, new repurposable drugs have been identified by network medicine approaches (see Joseph Loscalzo)
- Two randomized clinical trials were performed: One tested the use of CRP-guided steroid use (see Yewande Odeyemi) and another randomized different doses of anticoagulation (see Sanjum Sethi) in Covid-19 patients.
COVID-19 Rapid Response Grants in the News
-
Researchers explore how COVID-19 affects heart health in Black women
-
Additional $400K awarded for fast-tracked COVID-19 heart and brain health research
-
12 scientific teams redefining fast-tracked heart and brain health research related to COVID-19
-
$2.5 million now available for fast-tracked heart and brain focused scientific research of COVID-19
American Heart Association and the Global COVID-19 Pandemic
- Visit AHAjournals.org/Coronavirus for the AHA president’s statement, related journal articles, and other resources. Included on the site is a Circulation series of video interviews on best practices and insights from healthcare providers on the front lines, across the U.S. and around the world.